Wegovy reduced arthritis pain in randomized trial. Also: Will GLP-1s end bariatric surgery?
That and other stories in "Five on Friday" for November 1, 2024...
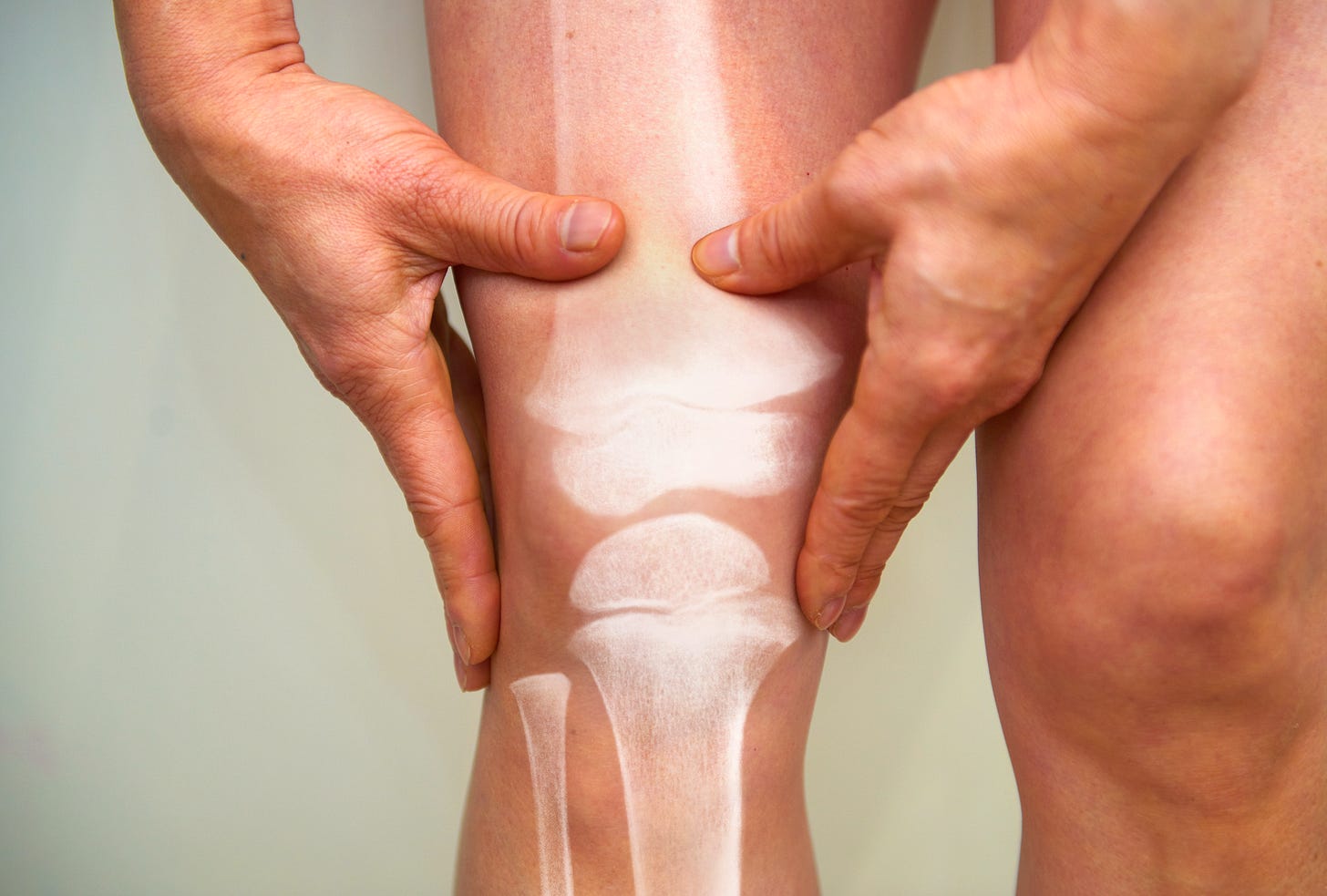
Item 1: Semaglutide (Wegovy) trial successfully treats arthritis knee pain. Are GLP-1s spelling the end of bariatric surgery?
In another win for semaglutide—the GLP-1 behind Ozempic and Wegovy—results from a randomized placebo-controlled trial released this week in the New England Journal of Medicine found remarkable improvements in knee pain and other related symptoms among patients with osteoarthritis and obesity.
The decreased pain and improved metrics on mobility were a trifecta: the findings were statistically significant, clinically relevant (i.e., genuinely noticeable differences on the patient level, not small changes that were only detectable in “big data”), and the improvements occurred within just a year. Participants had an average body mass index of over 40 when the study began, putting more than half of the participants in the severe obesity category.
Throughout the trial, participants in both groups received counseling on a reduced-calorie diet and physical activity—so the placebo group received more active care than they would have in real world conditions. That implies that the GLP-1s offer an even more impressive advantage.
While this study was funded by Novo Nordisk (the makers of Wegovy), the study is another impressive win. Some industry-funded studies are chock full of tricks and problems that make their drug look better than reality. That’s just not been the case so far with the GLP-1s. The outcomes have been clean, the effect sizes impressive, and the safety profile excellent. Also, the trial participants were 82% women (who are more likely to have osteoarthritis) and were from 21 nations around the globe.
Meanwhile, a new observational study in JAMA Network Open assessing 17 million US patients showed that in the last few years, as GLP-1 prescriptions have increased, bariatric surgeries like gastric bypass have decreased by 25%. That’s impressive given just a two-year study window (2022-2023).
Both of the above studies should cause economists to reconsider the cost-benefit analysis of GLP-1 drugs. Some economists have concluded that the cost effectiveness of GLP-1s may not yet be there for some patient groups, given the sticker price. As I’ve said before, I think they may be wrong for many patients because the benefits of the GLP-1s are still being worked out. Decreased pain in osteoarthritis patients may eventually lead to fewer knee surgeries (and fewer miserable and expensive complications, which I see as an ER doctor). It’s too soon to tell from the NEJM study, but it’s something we are watching.
On top of that, last week, Novo Nordisk reported by press release that its pill version of semaglutide decreased cardiovascular morbidity and mortality in its latest clinical trial. Pills would lower the costs, for sure. These drugs continue to amaze. What an important story this continues to be.
Item 2: Lassa Fever patient dies in United States.
The CDC reported a case of Lassa Fever in the United States this week. The infection occurred in West Africa and the patient then travelled back to Iowa, where they died. Lassa Fever is an RNA virus that circulates in West Africa. It causes around 100,00-300,000 cases per year, and has a case fatality rate of around 15%, although it’s likely much lower than this because it can cause mild illnesses that go undetected. Antivirals exist. Vaccines do not, but are in development. The disease is rare in the US (and elsewhere) because it spreads mainly through rodents that live in West Africa. Human-to-human transmission is possible, but not by casual contact. In other words, this story was highly interesting to infectious disease experts, but the risk to the global population (including the US) is very low.
Nobody asked, but if I had to guess where the patient traveled from, I’d say Nigeria. Why? Math. Lassa Fever is basically limited to four countries in West Africa (plus three others with occasional cases). Nigeria is one of those four and its population is 850% that of the other three combined. In any case, it doesn’t matter because this is probably an isolated or rare event that shouldn’t cause concern for us other than a reminder that there are people in more dangerous places who need basic medical necessities that we take for granted. Those tools save lives, be it from Ebola, Covid-19, or Lassa Fever.
Item 3: A pill that decreases spread of Covid-19?
Antivirals like Paxlovid and Molnupiravir ought to decrease transmission of SARS-CoV-2. The trials looking at this technically failed, but as I’ve written before, that may have been due to flaws in the study designs, rather than reflections of reality. (My optimism on this might be noteworthy to many of you because I tend to be fairly pessimistic about how useful these drugs really are in the post-vaccine era.)
There’s hope. And we like hope. A newer antiviral called Ensitrelvir (made by Shionogi, a drug company in Japan) was studied to see if household contacts of Covid-19 patients had lower rates of infection if they began taking the pill. We don’t have the raw numbers, but regulations require that drug companies give a public readout when data for the primary outcome of phase III randomized controlled trials become available to researchers. The company did that and released this information. So, we know that this pill decreased Covid-19 transmission in homes and that the statisticians were satisfied.
Given the size of the trial, we can guess that the effect size is possibly impressive. We know there were around 2,400 people in the study. Doing a little reverse engineering, we can estimate that to have reached statistical significance on decreased infections, the pills must have been good enough to have reduced infections by at least 25% (e.g. from 4% to 3%), but it’s more likely that they reduced far more infections than that. We’ll find out when they publish the data.
Ensitrelvir seems to be an interesting drug. Unlike Paxlovid, it has actually been shown to help patients with symptoms during the Omicron era (Pfizer’s study of Paxlovid for symptom resolution failed, which nobody seems to have noticed, despite it being in the New England Journal of Medicine). In a previously published randomized placebo-controlled trial of Ensitrelvir, however, symptoms improved sooner for participants who received the drug, albeit only by around a day (8 days versus 7 days). If the drug can decrease household transmission, we’ll want it—especially for high-risk households. The FDA has expressed interest in moving this drug through its approval process quickly, but it has not happened yet. Therefore, as of now, Ensitrelvir is not available in the United States.
Item 4: Vascular access in out-of-hospital cardiac arrest study results released.
When someone’s heart stops beating while they are outside of a hospital, the likelihood that they will survive depends on the cause, the overall health of the patient prior to the event, and whether they receive timely and effective CPR. Generally, the rates of recovery from out-of-hospital cardiac arrest are not high. So, we’re looking for ways to improve.
It surprises many people to learn that medications like epinephrine don’t seem to help outcomes in out-of-hospital cardiac arrests. But EMS teams do their best and gain vascular access in order to at least try (and adhere to American Heart Association guidelines). A new study in the New England Journal of Medicine looked at whether teams saved time and whether outcomes improved for such patients by drilling into patients bones (“intraosseous access” or “IOs”) rather than traditional IV catheters. If IO lines turned out to lead to faster administration of medications (e.g., calcium, a great antidote in cases of deadly levels of potassium in the blood), it was hoped that outcomes might improve.
This trial literally had EMS crews open opaque envelopes at the start of each case. Inside the envelopes were instructions indicating whether they should try regular IV catheters or IOs to get vascular access. Successful vascular access was achieved more often in the IO cases (92%) than the traditional IV cases (80%). But from there, no effect was seen. In both groups, the rate of survival a month later was similar (10%-12%), as were the rates of survival with favorable neurologic status (i.e., able to walk without assistance, take care of their own affairs, etc), albeit lower, at 8%-9%. (For anyone interested, here’s the neurologic outcome scoring system. “Favorable” was defined as a score of 0-3 in this study.)
In another trial published in NEJM this week, the route by which EMS crews gave medications did not matter either. In that one, favorable neurologic outcomes were even lower (<3%), for reasons which probably reflected the patient population more than anything else.
I was hoping that IOs would show a benefit in these trials. IOs seem quicker to me and easier to perform in hectic non-hospital settings. At least now EMS crews know that if they’re having any difficulty with traditional IV access, IO access is just as good an option.
What we’re left with, however, is that the most important things in CPR are not the medications, but early and continuous high-quality chest compressions and (when warranted) early defibrillation.
Item 5: Poll of the Week.
Here are the results from the last poll. Thanks for your votes! Not too surprising that the Inside Medicine audience is highly interested in additional boosters doses of the Covid-19 vaccine. A full 88% of you said you either will get the CDC’s recommended second dose later this season, or would do so if it were available to you.
Item 5a: Poll of the Week for this week!
Inside Medicine reader Nancy Westvang suggested this one. Election Day in the US is just four days away. Healthcare is a major voting issue. I know it’s hard to choose, but if you had to pick one issue….
That’s it. Your “Friday Five!” Questions? Comments? Please chime in!
Feedback! Have any ideas for next week’s Poll of the Week? Any great articles you have read elsewhere that you want to share with the Inside Medicine community?







Unfortunately, ensitrelvir, like Paxlovid, interferes with the metabolism of tacrolimus, a common anti-rejection med for transplant patients. The transplant community desperately needs an effective oral antiviral for Covid. (Remdesivir is the preferred antiviral but has to be given IV on three consecutive days. Molnupiravir is taken orally but is much less effective).
Hoping Ensitrelvir gets out of FDA limbo--it seems more effective than Paxlovid, currently cheaper and far less drug interactions. It would be nice to have another antiviral in the toolbox.