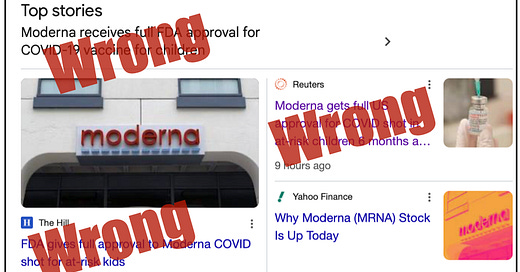
No, the FDA did not actually fully approve Moderna's pediatric Covid-19 vaccine yesterday.
Despite media headlines, a narrow approval was granted, which will make Moderna's vaccines far less available to children than they have been until now.
Today’s Inside Medicine is a deep dive that corrects the record on some inaccurate media coverage yesterday. If you want to support work like this…

You could be forgiven if you believed that the FDA granted full approval to Moderna’s Covid-19 vaccines for children yesterday. Indeed, that’s how many major media outlets portrayed things. After all, “FDA approves Moderna’s pediatric Covid-19 vaccine” headlines sound pretty promising, especially in an HHS run by Secretary Robert F. Kennedy Jr.
But full approval isn’t what happened at all. What actually happened is troubling…
The short version.
Rather than granting approval for all children starting at 6 months of age, the FDA limited its approval of Moderna’s pediatric Covid-19 vaccine to children known to have at least one high-risk underlying medical condition. That excludes the 54% of children ages 6-23 months who were hospitalized for Covid-19 in the last year despite having no known underlying medical conditions. Worse, the decision means that vaccines will be denied to children with risks yet to be identified—that is, dangerously untreated diseases. Moreover, it’s difficult to identify which infants—the highest-risk pediatric group—have the conditions that increase the risk of severe Covid during infancy, when the pediatric risk is highest. (The risk of severe Covid-19 is quite high in the first year of life; it then goes way down until later in adulthood.) In fact, infections like Covid-19 often unveil serious underlying pediatric medical conditions.
Limiting Covid-19 vaccines to the rather small subset of children with previously diagnosed high-risk conditions means that a vast majority of kids will no longer have access to vaccines early in life, which is when, epidemiologically, they need them most.
So while yesterday’s headlines seemed positive, the reality is not. Because the vaccines were previously available to all children under emergency use authorization, this so-called “full approval” would be better described as a ban for most children. Make no mistake, that is the stated intent of the FDA. Trump appointees now running the FDA simply do not believe Covid-19 is a problem for most children, meaning that vaccines are not indicated.
The long version. Or, how the FDA ignored the best science.
How did we get here? Until now, vaccines for all children under age 12 had been available under emergency use authorization (EUA) since 2022. That meant that, theoretically, all children had access to the vaccines—assuming the CDC recommended them, which, until recently, it did.
While access to the vaccines under an emergency use authorization regime has worked well, full FDA approval of the pediatric Covid-19 vaccines was eventually seen as a necessary step. (Moderna’s Covid-19 vaccine for adults was fully approved in January of 2021). After all, the FDA can easily revoke an emergency use authorization at any time, which was seen as an unsustainable risk.
Unfortunately, by limiting full approval to just children with high-risk conditions, the FDA has more-or-less done the opposite: it has removed the vaccine from the market for a majority of children, and almost all very young children in particular, the pediatric demographic that needs it most. Indeed, later in childhood, diagnosed risk factors like obesity start to crop up, but honestly, the risk that Covid poses to a 10-year-old with obesity is much, much lower than that to an apparently healthy 6-month infant.
So, while the FDA did not technically reject Moderna’s pediatric Covid-19 vaccine altogether—which, honestly, would not have shocked me, given the views of its current leadership—it basically did so for most of the very infants who need the vaccines the most: newborns without immunity.
How did the FDA reach this determination? We have no idea. Moderna’s large gold standard randomized, placebo-controlled clinical trial studied all children, not just those with known risk factors for severe Covid-19 illness.
The FDA knows Covid-19 epidemiology. It’s just ignoring it.
FDA leadership wants to believe Covid-19 does not pose a risk to healthy children. As we now know, that’s untrue.

How’d they get there? FDA leaders randomly decided that the risk faced by adults ages 50-64 is no longer high enough to warrant boosting. Since children ages 6-23 months old have the same rate of hospitalization as adults ages 50-64—a fact which surprises most people who believe kids don’t face serious Covid risks—FDA leadership leaped to the conclusion that kids do not need the primary vaccine series, an inference that was a complete non-sequitur. Why is that a non-sequitur? Because today’s 50-64 year olds have built up immunity to Covid through repeated vaccinations and infections. So, even if you were to believe that adults in the 50-64 age group no longer need routine boosting, it’s intellectually destitute to say that this means that infants in particular—who lack immunity because, hello, they’re new around here—no longer benefit from Covid vaccines.
But having made that leap, FDA leaders seem to have reasoned that if the youngest children don’t need vaccines, neither do older ones. (It’s true that the younger the child, the greater the Covid-19 risk.)
Many infants who would benefit from Covid-19 vaccines won’t be able to get them.
Let’s focus on infants ages 6-12 months. This is a key group because Covid-19 vaccines can’t be given until an infant reaches 6 months of age. The goal is to vaccinate children before they catch their first Covid-19 illness. The more time an infant spends in the world, the more likely a Covid infection becomes. I cannot state it any more clearly than this: every child deserves to have their first exposure to Covid-19 be from a safe vaccine, not a potentially unsafe virus.
For some reason, current FDA leadership still thinks it’s okay for 65-year-olds to get routine boosters, but that it’s not okay for all infants to receive the primary vaccine series—a group whose hospitalization rate rests squarely between the rates in 50-64-year-olds and 65-74-year-olds. Why are they drawing these lines, especially in the absence of any credible safety concerns for pediatric Covid vaccines? Well, we are living in arbitrary times, my friends. As a result, we now have annual flu vaccines approved for children starting at 6 months of age, but not Moderna’s primary series of the Covid vaccine. (Kids can still get Pfizer’s vaccine for now, but that’s likely to only be the case until Pfizer’s full “approval” application is granted.) In recent years, flu and Covid hospitalized similar numbers of kids. At a minimum, the FDA has not explained why it’s treating these pathogens differently.
Driving all of this is the temptation for some (including new FDA leaders) to blame pediatric Covid hospitalizations on underlying medical conditions and to pretend that really serious illness does not occur in kids. That appears to be a fantasy. Again, 54% of children hospitalized with Covid ages 6-23 months had no known high-risk conditions; 25% of the children hospitalized with Covid ages 6-23 months required intensive care unit admission.

Now, it’s possible that for most children (especially ones who have already been infected), that the economics aren’t favorable for all healthcare systems. But that's simply not the FDA’s purview. The FDA’s mandate is to determine whether vaccines are safe and effective. That’s it. No more, no less. Population recommendation policies are supposed to be determined by other agencies, like the CDC—albeit ideally not under the thumb of noted anti-vaxxers in Secretary Kennedy’s circle.
It bears mentioning that the 46% of children ages 6-23 months hospitalized with Covid-19 did have known medical conditions putting them at elevated risk of severe disease—and that this 46% probably comes out of a rather small subset of the pediatric population. But given the rates, that just means it’s extremely important to vaccine the small subset of babies with known Covid risks, whereas it might merely be reasonably-to-very important to vaccinate babies without such known risks. Just because the relative risk is off-the-charts for the highest-risk pediatric population does not mean that the baseline risk for all other children is low—they aren’t. I’m sure that someday AI and full-scale genomics will be able to accurately predict precisely which infants are at high risk of severe Covid-19. At that point, we could choose to vaccinate just those kids. That ability is years, if not decades, away.
Which leads back to the original sin of the new FDA leadership’s view on this. It is almost impossible to identify which of today’s newest crop of 6-month-old babies have conditions that increase the risk for severe Covid. In fact, those conditions become apparent to parents and pediatricians over time, and viruses like Covid-19 play a major role in that. Any frontline clinician with genuine experience in acute emergency care will tell you this. Viral infections that are more serious than would have been normally anticipated are frequently how doctors uncover serious medical problems like diabetes or serious heart conditions.
Meanwhile, skeptics who believe that Covid-19 is a non-issue for young children seem to want it both ways on this exact angle. They dismiss literature that suggests that the virus increases the risk of pediatric diabetes as a form of measurement bias. That is, they do not believe that Covid-19 is responsible for giving these kids diabetes. Instead, they believe that Covid-19 merely brought the problem to the surface. Okay, fine! But that just underscores the importance of vaccinating all infants, regardless of the presence or absence of high-risk medical conditions—so that they don’t present to hospitals with diabetic ketoacidosis (DKA), a life-threatening complication of diabetes often triggered by otherwise mild infections, and very often how previously undiagnosed diabetes first gets discovered.
Tired: Randomized clinical trials. Wired: Vibes.
While the application is not public, Moderna’s application for full FDA approval had to hinge on its randomized clinical trial, a large study which measured immune responses and adverse events in all kids, not merely ones with known risks.
This is a problem. First, the FDA has moved the goalposts. Why should vaccine manufacturers bother doing high-quality studies if the FDA is just going to ignore the results and cherry-pick parts it likes? Indeed, back in May, FDA officials announced new standards for vaccine approvals. But their rationale was not seriously vetted, was not subject to public comment, and apparently did not include the input of career experts at the agency. The document that announced the FDA’s new framework made a number of unsupported statements. Worse, it presented overt misinformation regarding the vaccine policies of some peer nations (which has now been corrected by experts I know who wrote letters to the editor that were just published.)
I’d love to get my hands on the FDA scientists’ actual decision memo on Moderna’s pediatric Covid-19 vaccine application for licensing. What did that document say? What science did it cite? Why is some real-world (“big”) data important, but not others? Did leadership override the workgroup’s recommendations? If so, on what grounds? This should all be available to the public—especially for an FDA that is enamored with its own supposed newfound transparency.
Come on boys, walk that walk! Let’s hash it out. I’m actually willing to change my mind on the merits of this decision, if the data are there. But none of us should accept a process that fails to share data, nor one that omits the rationale behind drastic changes in national policies that affect millions of people.
So, my message to FDA leadership is this: Show your work. I want to know why you think that infants with zero immunity to Covid-19 should not receive the vaccines when the gold-standard randomized, placebo-controlled clinical trials designed to measure immunity (and that were powered to detect rare harms down to 0.1%) achieved their pre-specified goals. Gold standard science is not the public taking your word for it. Gold standard science is high-quality data combined with genuine transparency.
That’s all for now. Thanks for supporting science, facts, and the actual American way. Please help the cause by sharing this work…
If you have information about any of the unfolding stories we are following, please email me or find me on Signal at InsideMedicine.88.




"So, my message to FDA leadership is this: Show your work."
It's doubtful that the FDA will respond to this substack post. You should submit a FOIA request.
One of my main current "beefs" with all this as Dr Faust mentioned is that the pharma comps won't do the big trials, etc because why waste the time and money when the FDA is already laying out its criteria. This is just the start too. The same will happen with flu vaxes & others. It will be slowly whittled to fewer & fewer eligible for the shots because the mantra of "the healthy don't need all these shots" or "too many shots for kids" before 5, etc. They will appear to still care by not taking away all the vaxes although who knows a year or two from now what it looks like. But it's starting down the path of less & less reliance on vaxes/therapeutics & just do wholesome living with a wrist monitor.