New Paxlovid trial flops.
A small but well-done study found no decrease in mortality among hospitalized patients in three countries.
Starting point: Paxlovid used to work very well.
In 2021, Paxlovid clearly decreased hospitalization and death among Covid-19 patients who had high risks of severe disease and who had not been vaccinated or previously infected. It’s a drug that saved lives—and would have saved many more if the vaccines had not beaten it to the punch.
The key thing to know is that Paxlovid’s blockbuster effect was found in people who had neither been infected with SARS-CoV-2 nor vaccinated. But by the time it actually reached the open market, the vast majority of the high-risk US population had already been infected or vaccinated. That immunity provides substantial long-term protection from severe Covid-19 when future infections occur, a fact which should not be minimized.
So, we’ve never really had the whole story on how well this drug works during its life in the real world. Observational studies (including work by my colleagues and me, and countless other studies) have helped, but we’ve been waiting for far more valuable trial data. In the last few months, we’ve gotten some news, finally.
It’s good news or bad news, depending on how you see things.
It’s 2024. Where do we stand?
Well, we already found out this spring that Paxlovid is not as useful as it once was. Pfizer finally released its results from its 2021-2022 trial showing that Paxlovid had no influence on symptoms among vaccinated patients with high risks or unvaccinated standard-risk patients. That study also found that hospitalizations were not statistically different across the Paxlovid and placebo groups but the study was not specifically designed to be definitive on that question.
But, we’ve been awaiting the results from a randomized controlled trial from the UK (the PANORAMIC study), which are expected to give key results on hospitalization and mortality for thousands of study participants.
New trial data: Paxlovid does not improve mortality in hospitalized patients.
In the meantime, we got some other data that is important. In late May, another UK-based study quietly preprinted findings from its randomized controlled trial of Paxlovid (conducted in 2022-2023 in the UK, Indonesia, and Nepal). In this study, patients who were being hospitalized with Covid-19 were randomized to either receive Paxlovid or not. The patients' mortality rates were compared after 28 days. There was absolutely no difference.
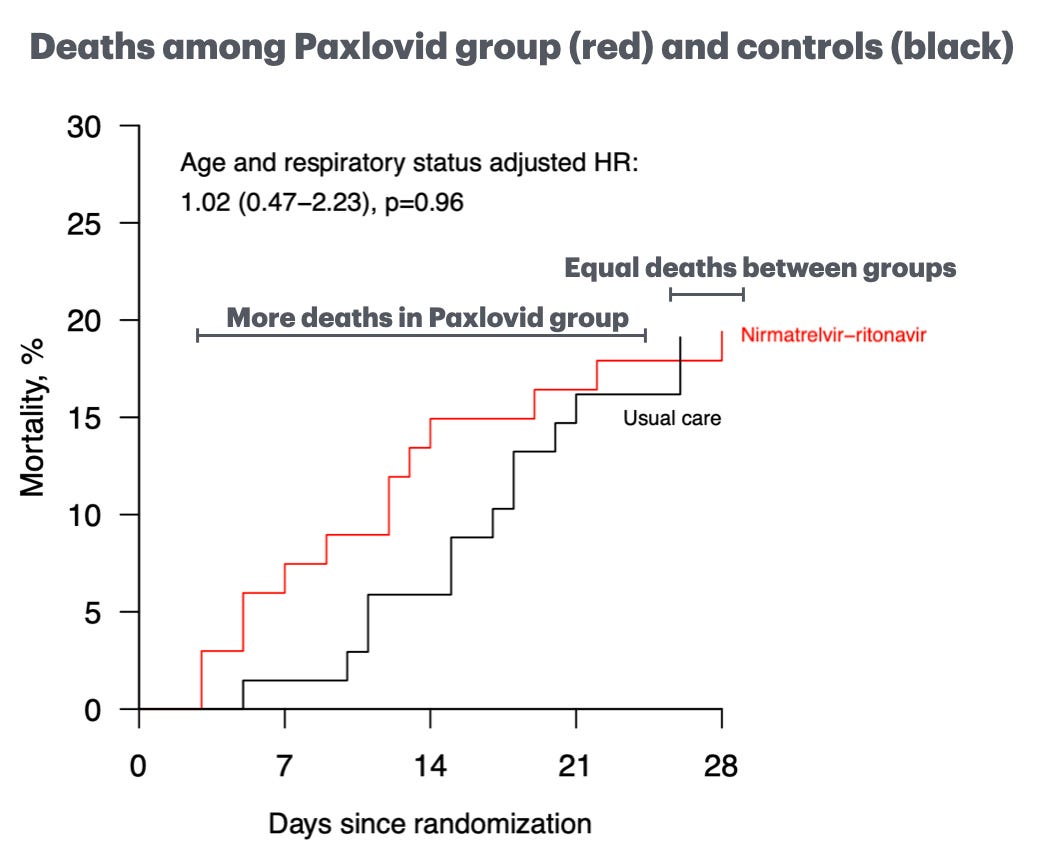
This figure is pretty jarring. If the study had looked at 14-day outcomes, there would have been more deaths in the Paxlovid recipients. By day 28, things were equal. In any case, not a win for Paxlovid.
I ran some math known as power calculations—albeit informally—that I want to share with you. As it stands, Paxlovid was 0% effective in preventing death among patients hospitalized for Covid in the new RECOVERY study.
But let’s imagine that we somehow knew for sure that the drug is effective, but that due to random chance, the study was “unlucky” and found that the death rate in the drug and control groups were equal?
In this table I made for us, the left column represents the “ground truth” hypothetical. That is, if Paxlovid were really 1%, 5%, 105, 25%, 50%, or 75% effective in decreasing mortality, how likely would the 50-50 result (or worse) that was seen in the RECOVERY trial be (right column)? Here’s the approximate readout:
In other words, if Paxlovid is actually 75% effective, the odds of a study like RECOVERY finding a 50-50 death rate (or worse) in the control and drug recipients just due to chance/bad luck is zero. If Paxlovid is actually 25% effective, the odds are just 18%. There’s a reasonable chance (40%-48%) that Paxlvoid could be 5-10% effective against death, despite the 50-50 mortality findings in this study.
Folks, that is not good news for this drug. Remember: Pfizer’s initial study found an 88% decrease in hospitalizations or deaths, and literally 100% of the deaths were among placebo recipients.
While the numbers in this study are on the smaller side, the researchers who did the study are highly respected and used good methods. For context, the parent study (RECOVERY) has randomized over 49,000 Covid-19 patients since 2020 to see which drugs work and which ones don’t. (It was this study, for example, that found that the inexpensive steroid dexamethasone had a massive benefit for Covid patients in 2020.)
In short, this study was small, but meaningful.
Is this the end for Paxlovid?
While this is a pretty hefty blow to Paxlovid, it is not the end of the story for this drug, for two reasons. First, the RECOVERY trial results looked at patients already being hospitalized. So, we do not know if Paxlovid might still decrease hospitalizations and death in patients who are earlier in their illness. That is what Pfizer’s 2021 blockbuster study found. The question is whether it remains true in 2024. The eagerly awaited PANORAMIC study will likely answer this question, as it is much larger and designed specifically to answer that question.
We should brace for the possibility that the PANORAMIC trial will either show Paxlovid is now completely unable to decrease hospitalizations or deaths, or that at best, it does so modestly for a narrow group of very high-risk patients. Results are anticipated this year.
Remember when I said “It’s good news or bad news, depending on how you see things.” What did I mean by that? Well, the bad news is that the drug doesn’t seem to work as well as it once did. The good news is the reason for this: Collectively, we have far more immunity to severe consequences of Covid than we did back in 2020-2021.
So, here’s the best way I can summarize things: It isn’t that Paxlovid no longer works; rather, it’s that in 2024, there are relatively few people who still seem to need really it to stay alive or out of the hospital.
What about Long Covid and Paxlovid?
News on that is expected soon…





I would LOVE to see a trial on Paxlovid used the way I use zinc for colds where it is started at the first hint of infection or when one knows they have been exposed. I take a specific type of zinc lozenge when I get that internal feeling that my body is fighting off a cold. If I do that for 24 hours, the cold never happens. If I am late in starting it and already have symptoms, the cold progress stops at that level and the cold duration is ½ what my normal is without zinc. IF Paxlovid is an antiviral, doctors and patients should not be waiting to start it. I know of soooo many times where drs said “wait til you have more symptoms.” That defeats the purpose. We know that long covid and post covid autoimmune diseases and organ damage is happening in a small % of patients. These are lifetime consequences for a small % of people, but a large number in total. I just had my first covid infection, most likely with the new variant FLiRT, and it was a full flu with severe disabling fatigue for 4 days. I was not able to get Paxlovid until I was at the end of my second day of infections because the dr kept saying no without explaining why. I eventually found out it was for some drugs on my med list that I no longer take or only take occasionally and was willing to skip. It was too late for Pax to do any good. I was sick for two weeks and didn’t test negative until day 16. I had to reschedule two important dr appts that now will take me 4 months to get back in. And I was stuck out of town for 5 days which was very expensive and uncomfortable vs being sick at home. Covid still is a major disease that deserves more attention on research and doctors who take more time to consider treatments. I am 62 with 3 issues that potentially could have been longer term consequences. I seem to have averted them for now, but still run a risk a new autoimmune disease will pop up that wouldn’t if I had not had a major virus. I eagerly await the long covid study, which is why I spent the money on Paxlovid despite knowing my dr’s delay meant I wasn’t being helped by Pax on current symptoms. I had the spring booster 3 weeks before the infection. It did not prevent the infection nor did it reduce the symptoms to a tolerable level. 7 people that we know of caught the virus from my family and all but one had flu like symptoms, not a minor cold.
Have studies been done regarding Paxlovid's affects on symptoms? It seems to have worked well in my case.